Energy Storage
About Energy Storage
Energy storage is a key enabling technology in the exploitation of intermittent renewable energy resources such as wind and solar, meeting the fluctuations in demand. It is also critical for mobile devices from cell phones to vehicles.
Among various storage techniques, hydrogen and batteries have been advocated as promising media to store energy.
Hydrogen has the highest energy per mass of any fuel and the energy stored can be extracted using hydrogen fuel cells at high efficiencies while releasing no pollution. These have been demonstrated in passenger vehicles, buses, and forklifts. The low ambient temperature density of hydrogen, however, results in a low energy per unit volume. Conventional compression and liquefaction methods have inherent problems.
For batteries, electricity is stored and then released upon demand. There are many different types of batteries featuring distinct advantages related to specific applications. For mobile applications, the current mainstream Li ion batteries have high energy density. For load leveling related to electrical grid, batteries using naturally abundant elements such as Na and Mg are under intense investigation.
We work under non-disclosure agreements and don’t hold any IP on the processes developed under such agreements. Our objective is to build close partnerships with companies in order to solve your technical problems and to deliver high quality products. Ensuring the quality of our products is important to Boron Molecular.
We operate an ISO9001 quality system and we have an in house analytical laboratory that can perform a variety of analytical tests. We are currently supporting customers in a wide range of fields ranging from paints, coatings, adhesives and electronics through to cosmetic, veterinary and pharmaceutical industries.
Hydrogen Storage Compounds
Materials-based hydrogen storage enables high volumetric densities and gravimetric densities of hydrogen. Challenges, however, remain related to developing compounds that simultaneously have high hydrogen capacities, low dehydrogenation temperature, controllable H2 release kinetics and good regeneration capability. Boron Molecular is teaming up with the University of Wollongong to help the research community to search for the right candidates by enabling them to readily access high quality hydrogen storage compounds.
Sodium octahydrotriborate (NaB3H8) (BM1607)
Empirical Formula: NaB3H8
Molecular Weight: 63.4
Description:
White powder. Decomposes around 100 ºC under inert atmosphere. Slowly reacts with water (less than 10 % in one week at ambient temperature). Highly hygroscopic. Highly soluble in most ethereal solvents.
Application
Sodium octahydrotriborate (NaB3H8) is a salt with high hydrogen content. It has a high solubility and reasonable stability in water. A rapid release of high purity H2 gas can be obtained through hydrolysis facilitated by transition metal based catalysts.
The high Na to B ratio leads to the formation of complex polyborate mixture during hydrolysis, and this metastable solution is difficult to crystallize (Figure 1). Compared to NaBH4 and NH3BH3, both of which tend to form hydrolytic products with low solubility in water, NaB3H8 can provide a greater storage capacity of hydrogen while maintaining a liquid phase. Liquid phase hydrogen storage has better compatibility with the current liquid fuel dispersion techniques.
Fig. 1 The formation of ployborates during the hydrolysis of NaB3H8.
The bulky B3H8 anion also makes it an ideal source to make hydrogen-rich ionic liquids.
Different from NaBH4, NaB3H8 is highly soluble in most ethereal solvents, including diethyl ether, and therefore can be an effective reducing agent in organic synthesis.
It is an important precursor to other metal and nonmetal octahydrotriborates, whose high reactivity has made them interesting for the fabrication of boride thin films.
References:
1. Z. Huang, G. King, X. Chen, J. Hoy, T. Yisgedu, H. Lingam, S. Shore, P. Woodward, J. Zhao. “A Simple and Efficient Way to Synthesize Unsolvated Sodium Octahydrotriborate.” Inorganic Chemistry, 2010, 49, 8185.
2. Z. Huang, X. Chen, T. Yisgedu, J. Zhao, S. Shore. “High-capacity hydrogen release through hydrolysis of NaB3H8.” International Journal of Hydrogen Energy, 2011, 36, 7038.
3. D. Schubert, D. Neiner, M. Bowden, S. Whittemore, J. Holladay, Z. Huang, T. Autrey, “Capacity Enhancement of Aqueous Borohydride Fuels for hydrogen storage in liquids”, Journal of Alloys and Compounds, 2015, 645, S196.
4. W. Chen, Z. Huang, G. Wu, T. He, Z. Li, J. Chen, Z. Guo, H Liu, P. Chen, “Guanidinium octahydrotriborate: an ionic liquid with high hydrogen storage capacity”, Journal of Materials Chemistry A, 2015, 3, 11411
Na-ion Battery Electrolytes
Owing to the low cost and high natural abundance of sodium (Na), Na-ion batteries (NIBs) have been extensively studied very recently. The most common electrolyte formulation for NIBs is NaClO4 or NaPF6 dissolved in carbonate solvents such as ethylene carbonate and propylene carbonate. NaClO4 is potentially explosive, however, and NaPF6 is sensitive to moisture, evolving highly corrosive HF. Since NIBs have been largely considered for stationary energy storage due to its lower power density, and the deployment of NIB stacks would normally require a large quantity of electrolyte, an electrolyte that is both highly safe and efficient is critical. Boron Molecular is teaming up with the University of Wollongong to help the research community to provide alternative candidates by enabling them to readily access alternative high quality electrolyte formulations.
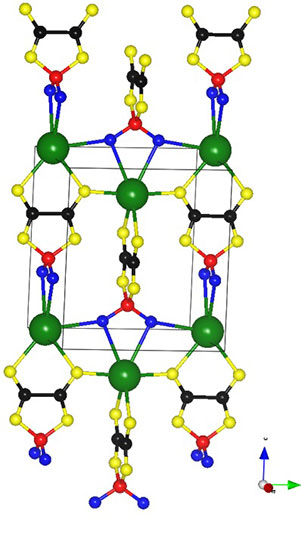
Sodium–difluoro(oxalato)borate (NaDFOB)
Empirical Formula: C2BF2NaO4
Molecular Weight: 143.77
Description: White powder. Slowly reacts with water.
Application
Sodium difluoro(oxalate)borate (NaDFOB) is an electrolyte salt for Na-ion batteries. The electrolytes prepared by dissolving this compound in carbonate-based solvents have a wide electrochemical stability window, low viscosity, and good conductivity. It does not yield HF when reacted with water. These could improve the safety of the future Na-ion batteries, which are expected to be used for large-scale electricity storage.
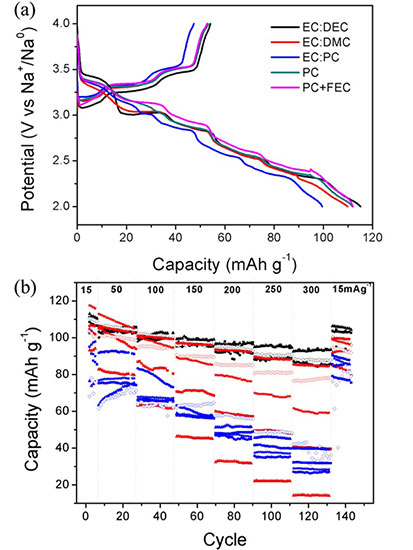
Compared with NaClO4 and NaPF6 which are commonly used in the literature, this compound enables better compatibility with a couple of most studied compounds for Na-ion batteries, such as carbonaceous materials, TiO2, and Prussian blue, and therefore better rate capability and cycling performance. Na-ion batteries are believed to hold promises for large-scale energy storage such as smart-grid where the market has been predicted to be well over billions of dollars per year, NaDFOB could help the development of reliable batteries to meet the great demand for sustainable energy.
Reference:
1. J. Chen, Z. Huang, C. Wang, S. Porter, B. Wang, W. Lie, H. K. Liu, “Sodium-difluoro(oxalato)borate (NaDFOB): A new electrolyte salt for Na-ion batteries”, Chemical Communications, 2015, 51, 9809.



